In order to safety store and transport hydrogen with greater density needs to be combined with another chemical to enhance stability. Many options have been explored and are generalised into three categories.
- Metal organic frameworks (MOF)
- Metal hydrides
- Chemical hydrides
Each chemically enhanced method has its own advantages and disadvantages. For example MOF are excellent materials for absorption of H2, but demonstrate difficulties in consistent release. While metal hydrides feature a metal-hydrogen bond, are dense, but highly reactive and are explosive upon contact with moisture or oxygen in the atmosphere. Chemical hydrides store hydrogen both as a proton and a hydride, but not reactive upon release in the atmosphere. A comparison of the gravimetric densities versus volumetric densities reveals one chemical hydride that satisfies the DOE (US department of energy) requirement as a replacement for compressed hydrogen and petroleum based fuels. Below is chart produced by the DOE for different hydrogen containing materials, including gaseous and liquid hydrogen.
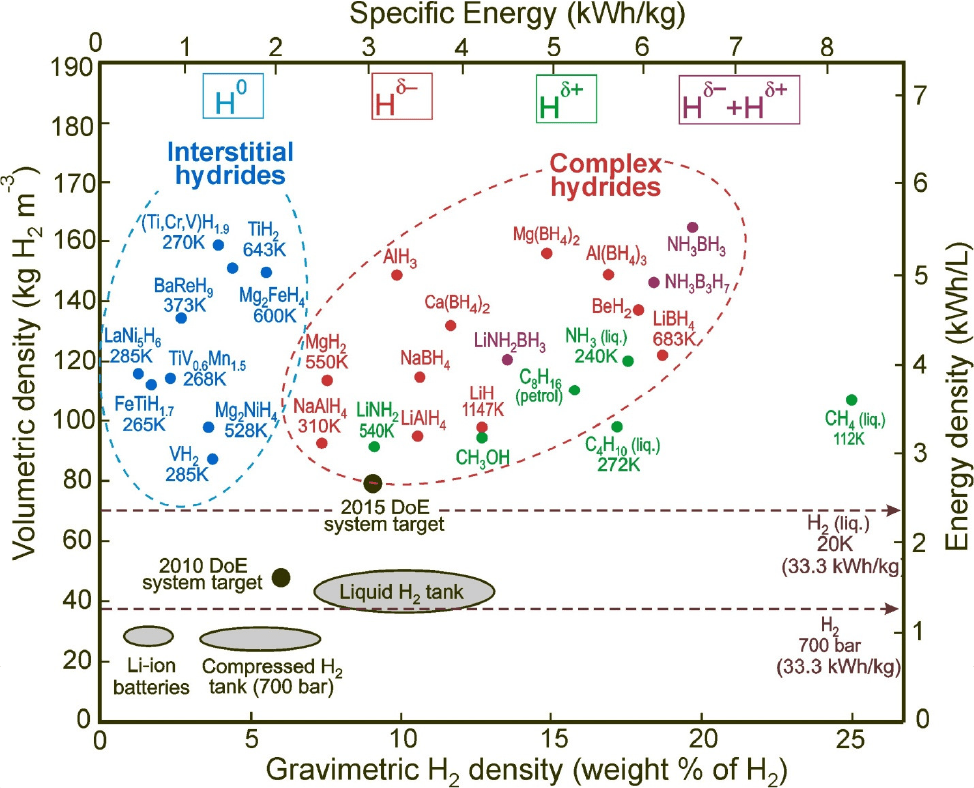
Figure reproduced from Edwards and Kuznetsov, Chapter 4. Turning Points in Solid State Materials and Surface Science. RSC Publishing, 2008.
The above map reveals the high hydrogen carrying potential of ammonia borane (highlighted). See a more detailed map here. Another consideration is the temperature (energy) required to release the hydrogen from the compound. Again, ammonia borane (AB) has very favourable properties. Ammonia borane is the combination of ammonia (NH3) and borane (BH3). It has one of the highest hydrogen content for a small molecule (19.6% wt) when 3 equivalents of H2 are released. But generally only 1 to 2.5 equivalents of H2 released.
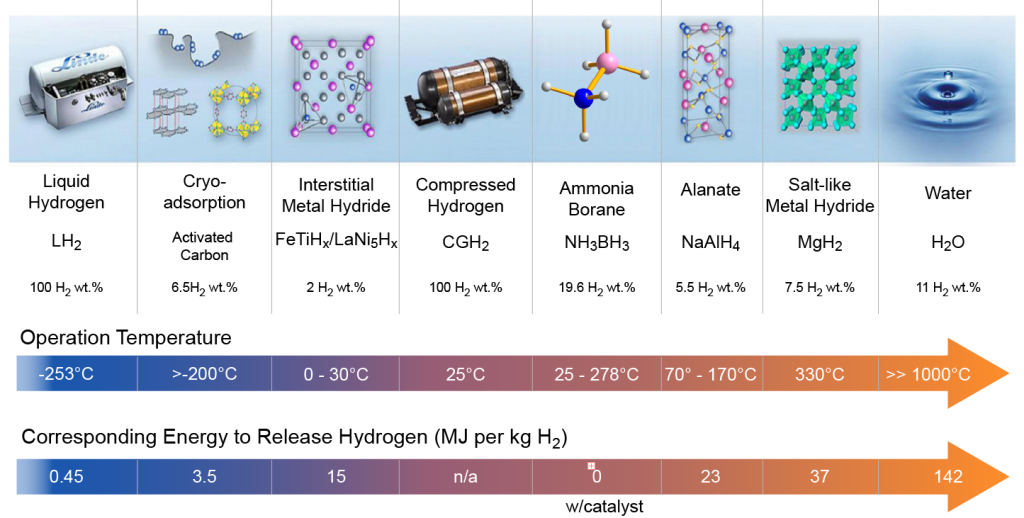
Figure reproduced from Kaneez, et al. JCCS, 10.1002/jccs.202200039.
Ammonia borane is colourless powder that is soluble in polar solvents, but only slightly soluble in Et2O and not soluble in toluene or pentane. It has a low toxicity.
Ammonia borane is synthesised from a variety of methods, but the most common is the reaction between ammonia carbonate/sulphate and sodium borohydride in tetrahydrofuran solvent. After filtration, the ammonia borane is obtained in greater than 90% yield.
To read about borax, the basic building block of ammonia borane and what reserves of boron are available.
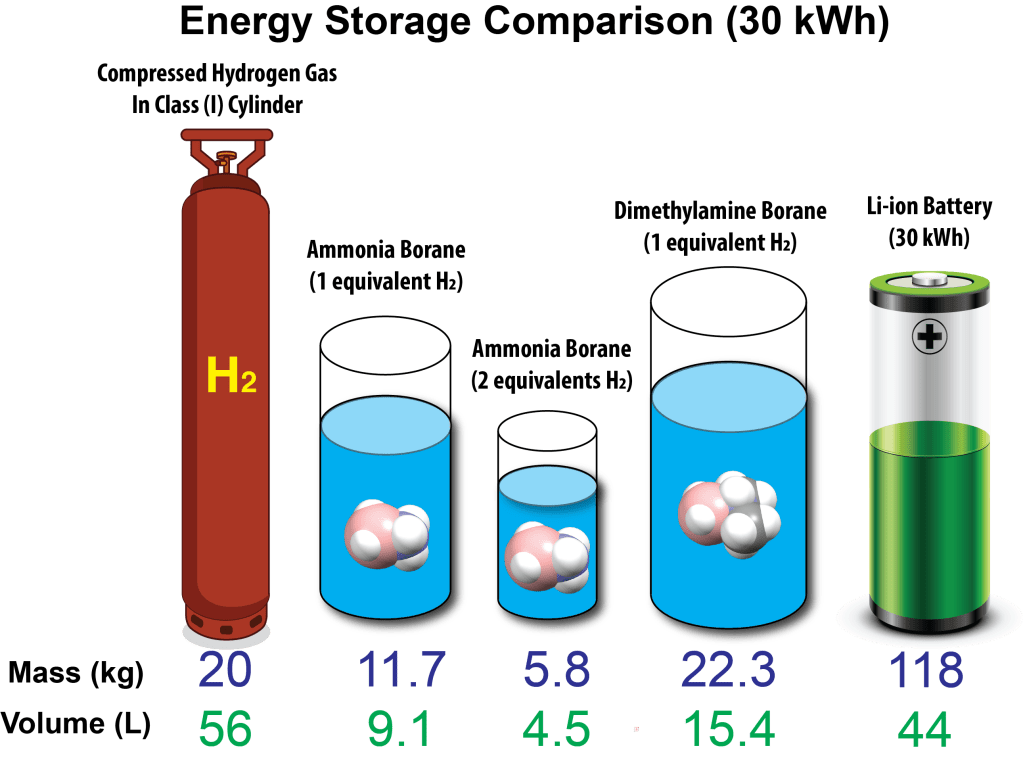
There are several methods to release hydrogen from ammonia borane including direct thermolysis (heating), catalytic hydrolysis or direct catalysis. Thermolysis is undesirable as it produces borazine, a highly volatile PEM fuel cell poison. Instead a purposely designed catalyst can be employed to liberate H2 from ammonia borane. Proceed to technology.
Goto the Technology page